6.7 Change the secondary structure of a peptide
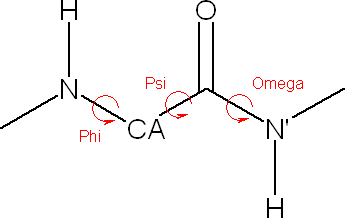
VEGA ZZ is able to change the secondary structure of a peptide moving the
Phi, Psi and Omega torsions. To show the dialog window, you must select Edit
![]() Change
Change
![]() Secondary struct. in main menu.
Secondary struct. in main menu.
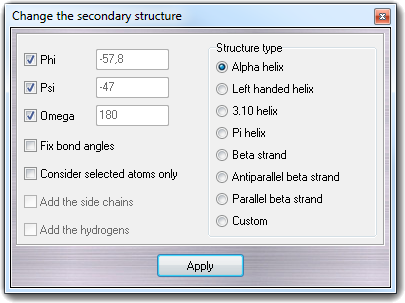
The Structure type box allows to specify the secondary structure type: several pre-defined structure types are already defiend (Alpha helix, Left handed helix, 3.10 helix, Pi helix, Beta strand, Antiparallel beta strand and Parallel beta strand) but you can create custom structures selecting Custom and changing the Phi, Psi and Omega torsion values:
The checkboxes at the left of each torsion name allow to enable/disable which
angles are changed to the characteristic secondary structure value, eventually
preserving the original torsions. Checking Fix bond angles, the bond
angles distorted by the rotation of the torsions are reverted to the canonical
value and checking Consider selected atoms only, the torsion modification
is applied to the selected/visible atoms only, keeping the other atoms
unchanged.
Add the side chains and Add the hydrogens checkboxes are
intentionally disabled and are operative when you build a peptide from its
primary structure (for more information, see how to
build a peptide). Clicking Apply button, the secondary structure
of the peptide
in the current workspace is changed.
WARNING:
the routine changing the torsion angles is working outside the rings. In other
words, it's unable to change the secondary structure of cyclic peptides,
including peptides with disulfide bridges. To avoid this problem, you could
temporally open the ring breaking one bond (e.g. a disulfide bridge or a
backbone bond), change the secondary structure and, if it's possible, rebuild
the broken bond.
The following table shows Phi, Psi and Omega values of the most common secondary structures:
| Secondary structure | Phi | Psi | Omega |
| Alpha helix | -57.8 | -47.0 | 180.0 |
| Left helix | 7.8 | 47.0 | 180.0 |
| 310 helix | -74.0 | -4.0 | 180.0 |
| Pi helix | -57.1 | -69.7 | 180.0 |
| Beta strand | -135.0 | 135.0 | 180.0 |
| Antiparallel beta strand | -140.0 | 135.0 | 180.0 |
| Parallel beta strand | -120.0 | 115.0 | 180.0 |